Purchasing over $3,500? Contact us for further discounts. Over 250,000+ Products Available and Growing!
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseImplantsIncontinenceIndicators and SignageInstrumentsI.V. TherapyKits, Packs & TraysLabelsMannequins and ModelsMasksMetal/Plastic/PaperMicroaire Surgical InstrumentsNeedles and SyringesNurse Assist
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseConMed LinvatecCorneal ShieldCoretex
DeRoyalDevilbissDonovan IndustriesDraegerDynarexEndorphinExel InternationalFabrication EnterprisesFisher Scientific
MedlineMedtec MedicalMedtronics-SurgicalMedtronics - XomedMoore MedicalNascoNeotech Products, INC.New World ImportsNor-Lake ScientificNorth Coast MedicalNu-Hope LaboratoriesOhio Medical
Pall Life SciencesPatterson MedicalPhilips HealthcareR&B Wire Products:Industrial Laundry & Linen Transportation EquipmentRemington MedicalRespironics


RX Drugs order by EMAIL ONLY
We only sell to businesses, clinics, government facilities, individuals may contact us by email - info@go3a.com
Item#: Hos-490034-PK
$231.81
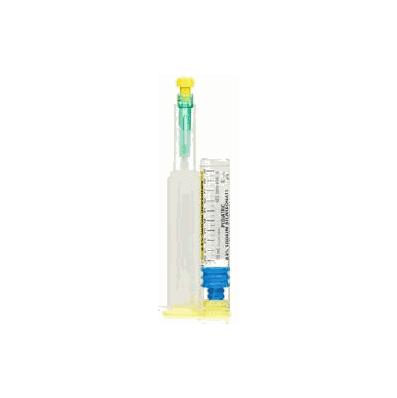
Alkalinizing Agent, Prefilled Syringe, 8.4%, 1 mEq / mL, 10 mL, Pediatric Intravenous Injection (10/Pk) by Hospira
AVAILABLE AGENTS:
Prefilled Syringe, 8.4%, 1 mEq / mL, 50 mL, click here
Prefilled Syringe, 4.2%, 0.5 mEq / mL, 10 mL, click here
Single Dose Vial, 8.4%, 1 mEq / mL, 50 mL, click here
Single Dose Vial, 4%, 0.48 mEq / mL, 5 mL, click here
Single Dose Vial, 5 mEq / mL, 10 mL, click here
- PRODUCT DETAILS
- GOT QUESTIONS
- RECENTLY VIEWED
Price shown is sold as PK/10
| Dealer # | 336858 |
| Manufacturer # | 490034 |
| Manufacturer | Hospira |
| Application | Alkalinizing Agent |
| Container Type | Prefilled Syringe |
| Dosage Form | Injection |
| Generic Drug Code | 02752 |
| Generic Drug Name | Sodium Bicarbonate, Preservative Free |
| NDC Number | 00409-4900-34 |
| Prop 65 | No |
| Storage Requirements | USP Controlled Room Temperature |
| Strength | 8.4%, 10 mEq / 10 mL |
| Type | Intravenous |
| User | Pediatric |
| Volume | 10 ML |
Features
- LifeShield™ Abboject™ glass syringe with male luer lock adapter and 20 gauge protected needle
- Sodium Bicarbonate Injection, USP is a sterile, nonpyrogenic, hypertonic solution of sodium bicarbonate (NaHCO3) in water for injection for administration by the intravenous route as an electrolyte replenisher and systemic alkalizer
- The BEST WAY to contact is by EMAIL - Use our "Contact Us" page, if you need immediate response, write URGENT on the subject line.
- Emails are monitored 9 am - 4 pm, Mon-Fri, except Holidays, California Time.
- Can't find the Exact Size? Contact us ( use "contact us" page) and we will get you the info.
- Need it Yesterday? we have options for 2-3 days, 4-5 days use the check out page shipping options. Take note: the clock begins the day after the order and excludes week-ends and Holidays.
- Can't Find the Exact Item but you found the Manufacturer? Send us the Info, and we will locate it for you.
- Need a Quote? Please email us, be accurate with part#, description and quantities.
- Overseas Customers? Contact us first before making your purchase.