Purchasing over $3,500? Contact us for further discounts. Over 250,000+ Products Available and Growing!
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseImplantsIncontinenceIndicators and SignageInstrumentsI.V. TherapyKits, Packs & TraysLabelsMannequins and ModelsMasksMetal/Plastic/PaperMicroaire Surgical InstrumentsNeedles and SyringesNurse Assist
AAAAllied HealthcareAmvexAnesthesia AssociatesArrow InternationalAspen Surgical/NeedlesBaxaorationBayerB&BBeckman CoulterBerry PlasticsBionixCandela/LUNARClinical LaboratoryCliniqaCommon SenseConMed LinvatecCorneal ShieldCoretex
DeRoyalDevilbissDonovan IndustriesDraegerDynarexEndorphinExel InternationalFabrication EnterprisesFisher Scientific
MedlineMedtec MedicalMedtronics-SurgicalMedtronics - XomedMoore MedicalNascoNeotech Products, INC.New World ImportsNor-Lake ScientificNorth Coast MedicalNu-Hope LaboratoriesOhio Medical
Pall Life SciencesPatterson MedicalPhilips HealthcareR&B Wire Products:Industrial Laundry & Linen Transportation EquipmentRemington MedicalRespironics


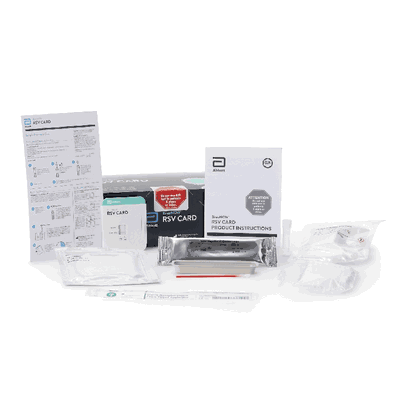
Respiratory Test Kit, Respiratory Syncytial Virus Test (RSV) Infectious Disease Immunoassay Rapid Test Card Format, sold as 22 Tests/Kit by BinaxNOW, Abbott Rapid Dx 430122
Contents Include:
(22) Test Cards, Transfer Pipettes, Positive Control Swab, Negative Control Swab, Elution Solution, NP Patient Swabs, Product Insert, Procedure Card
We can only ship to Businesses and Clinics.
Item#: Inv-430-122-KT
$484.36
- PRODUCT DETAILS
- GOT QUESTIONS
- RECENTLY VIEWED
| Dealer # | 564548 |
| Manufacturer # | 430122 |
| Brand | BinaxNOW® |
| Manufacturer | Abbott Rapid Dx North America LLC |
| Country of Origin | United States |
| Application | Respiratory Test Kit |
| CLIA Classification | CLIA Waived |
| CLIA Classified | CLIA Waived |
| Contents 1 | (22) Test Cards, Transfer Pipettes, Positive Control Swab, Negative Control Swab, Elution Solution, NP Patient Swabs, Product Insert, Procedure Card |
| Number of Tests | 22 Tests |
| Reading Type | Visual Read |
| Sample Type | Nasopharyngeal Swab / Nasal Wash Sample |
| Specialty | Immunoassay |
| Test Format | Test Card Format |
| Test Kit Type | Rapid |
| Test Method | Lateral Flow Method |
| Test Name | Respiratory Syncytial Virus Test (RSV) |
| Test Type | Infectious Disease Immunoassay |
| Time to Results | 15 Minute Results |
| UNSPSC Code | 41116126 |
Features
- Alere BinaxNOW® RSV Card is an in vitro rapid immunochromatographic assay used to detect respiratory syncytial virus (RSV) fusion protein antigen in nasal wash and nasopharyngeal (NP) swab specimens from symptomatic patients
- This test is intended for in vitro diagnostic use to aid in the diagnosis of respiratory syncytial virus infections in neonatal and pediatric patients under the age of 5
- Retrospective nasal wash Sensitivity: 89% Specificity: 100%
- Prospective NP swab Sensitivity: 93% Specificity 93%
- The BEST WAY to contact is by EMAIL - Use our "Contact Us" page, if you need immediate response, write URGENT on the subject line.
- Emails are monitored 9 am - 4 pm, Mon-Fri, except Holidays, California Time.
- Can't find the Exact Size? Contact us ( use "contact us" page) and we will get you the info.
- Need it Yesterday? we have options for 2-3 days, 4-5 days use the check out page shipping options. Take note: the clock begins the day after the order and excludes week-ends and Holidays.
- Can't Find the Exact Item but you found the Manufacturer? Send us the Info, and we will locate it for you.
- Need a Quote? Please email us, be accurate with part#, description and quantities.
- Overseas Customers? Contact us first before making your purchase.